402
Views & Citations10
Likes & Shares
TB has been a significant global health concern for many years, with millions of new cases reported annually. It is a leading cause of illness and death worldwide, particularly in low- and middle-income countries. Factors such as poverty, overcrowding, malnutrition, and weakened immune systems (such as those with HIV/AIDS) contribute to the increased susceptibility to TB.
The disease can manifest in two forms: latent TB infection (LTBI) and active TB disease. In LTBI, the bacteria are present in the body but are in a dormant state and do not cause any symptoms. However, they can become active and cause disease in the future if the immune system weakens [3,4]. Active TB disease occurs when the bacteria become active and multiplies, leading to the characteristic symptoms of TB.
Common symptoms of active TB include persistent cough (sometimes with blood-tinged sputum), fatigue, weight loss, fever, night sweats, and chest pain. However, symptoms may vary depending on the site of infection in extrapulmonary TB cases [5].
Diagnosis of TB involves various tests, including a medical history evaluation, physical examination, chest X-rays, and sputum tests to detect the presence of TB bacteria. Additional tests, such as blood tests and imaging studies, may be conducted to determine the extent of the infection and assess the response to treatment.
The treatment of tuberculosis (TB) typically involves a combination of antibiotics taken over a prolonged period. The specific treatment regimen depends on factors such as the type of TB infection (latent or active) and whether the bacteria are drug-sensitive or drug-resistant [6,7].
TREATMENT OF TUBERCULOSIS
Treatment of TB typically involves a combination ofantibiotics taken over a prolonged period. The specific regimen depends on factors such as the type of TB infection (latent or active) and whether the bacteria are drug-sensitive or drug-resistant [8]. Treatment aims to cure the infection, prevent the spread of TB to others, and reduce the risk of relapse.
Prevention of TB involves several strategies, including early detection and treatment of active cases, vaccination (with the Bacillus Calmette-Guérin or BCG vaccine in some regions), infection control measures in healthcare settings, and addressing social and economic factors that contribute to TB transmission.
Efforts to control and eliminate TB are ongoing worldwide, with organizations like the World Health Organization (WHO) leading global initiatives [9,10]. These initiatives include increasing access to accurate diagnosis, effective treatment, and preventive measures, as well as research and development of new tools and strategies to combat TB.
Latent TB infection (LTBI) treatment
LTBI refers to a state where a person has been infected with the TB bacteria but does not have active TB disease.
Treatment aims to prevent the bacteria from becoming active and causing disease in the future.
The most common regimen for LTBI treatment is a course of isoniazid (INH) taken daily for 9 months. Alternative regimens may involve taking INH with rifapentine once a week for 12 weeks or INH with rifampin daily for 3-4 months [11].
Active TB disease treatment
Active TB disease requires a combination of antibiotics to effectively kill the bacteria and prevent the development of drug resistance.
The most common initial treatment regimen for drug-sensitive TB includes a combination of four antibiotics: isoniazid, rifampin, pyrazinamide, and ethambutol. This combination is typically taken for an intensive phase of 2 months, followed by a continuation phase of 4-7 months with isoniazid and rifampin [12,13].
Depending on the circumstances, the treatment duration may vary. For example, if the TB infection involves the lungs, the continuation phase typically lasts for 4 months. However, if the infection affects other parts of the body (extrapulmonary TB), the continuation phase may extend to 7 months or longer.
It's crucial to complete the full course of treatment as prescribed by the healthcare professional, even if symptoms improve. Stopping treatment prematurely can lead to treatment failure, recurrence, and the development of drug-resistant strains [14-17].
Drug-resistant TB treatment
Drug-resistant TB refers to strains of TB bacteria that are resistant to one or more of the first-line antibiotics used for TB treatment. Treatment for drug-resistant TB requires a more complex regimen with additional drugs, such as fluoroquinolones (e.g., levofloxacin, moxifloxacin), injectable antibiotics (e.g., kanamycin, amikacin), and other second-line antibiotics. The duration of treatment for drug-resistant TB is longer, typically lasting for 18-24 months or more, and may involve multiple phases.
The management of drug-resistant TB is highly specialized and requires close monitoring and expertise from healthcare professionals experienced in treating this form of TB [18-20].
DEVELOPMENT OF FLUOROQUINOLONE [21-25]
Fluoroquinolones, a class of antibiotics, were developed through a process of scientific research and pharmaceutical innovation. The development of fluoroquinolones involved several key milestones and contributions over the years. Here is a general overview of the development of fluoroquinolones:
Discovery of Quinolones: The history of fluoroquinolones can be traced back to the discovery of quinolones, a class of antibiotics that preceded them. Quinolones were first developed in the 1960s and were effective against a range of bacterial infections.
Introduction of Fluoroquinolones: The addition of a fluorine atom to the quinolone structure led to the development of fluoroquinolones, which exhibited enhanced antibacterial activity and broader spectrum of coverage against both Gram-positive and Gram-negative bacteria. The first fluoroquinolone, nalidixic acid, was introduced in the 1960s and had activity primarily against Gram-negative bacteria.
Structural Modifications: Over time, researchers made various structural modifications to fluoroquinolones to improve their effectiveness, pharmacokinetics, and tolerability. These modifications led to the development of second-generation fluoroquinolones, such as ciprofloxacin and ofloxacin, which were introduced in the 1980s and offered improved coverage against Gram-negative bacteria.
Third and Fourth Generation Fluoroquinolones: Further refinements in the structure and properties of fluoroquinolones led to the development of third-generation (levofloxacin, moxifloxacin) and fourth-generation (gemifloxacin) fluoroquinolones. These newer generations provided expanded coverage against both Gram-positive and Gram-negative bacteria, including some drug-resistant strains.
Regulatory Approval: As each new fluoroquinolone was developed, it underwent rigorous testing and evaluation to demonstrate its safety and efficacy. Regulatory agencies, such as the U.S. Food and Drug Administration (FDA) and other international bodies, reviewed the scientific data and granted approval for the use of specific fluoroquinolones in the treatment of various bacterial infections.
Continued Research and Development: Ongoing research and development in the field of fluoroquinolones have focused on optimizing the pharmacological properties, expanding the spectrum of activity, and addressing emerging issues such as drug resistance and side effects. This has led to the introduction of newer fluoroquinolones and variations in dosage forms to improve patient convenience and compliance.
Fluoroquinolones have evolved over time, and the availability and use of specific fluoroquinolones may vary across countries. Additionally, concerns about the potential side effects and risks associated with fluoroquinolones have led to regulatory actions and warnings from health authorities. Therefore, healthcare professionals carefully consider the benefits and risks when prescribing fluoroquinolones and adhere to prescribing guidelines.
Overall, the development of fluoroquinolones represents a significant advancement in the treatment of bacterial infections, offering a broad-spectrum option for various conditions.
CLASSIFICATION OF FQs [26,27]
Fluoroquinolones can be classified into several generations based on their development and properties. Here is a general classification of fluoroquinolones:
First-generation fluoroquinolones: The first-generation fluoroquinolones include nalidixic acid. While nalidixic acid was the first fluoroquinolone to be developed, it has limited activity against Gram-negative bacteria and is mainly used for the treatment of urinary tract infections.
Second-generation fluoroquinolones: The second-generation fluoroquinolones are broader-spectrum antibiotics compared to the first generation. Examples of second-generation fluoroquinolones include ciprofloxacin, ofloxacin, and norfloxacin. These antibiotics have improved activity against Gram-negative bacteria and expanded coverage against certain Gram-positive bacteria.
Third-generation fluoroquinolones: The third-generation fluoroquinolones, such as levofloxacin, moxifloxacin, and gatifloxacin, exhibit enhanced antimicrobial activity against both Gram-positive and Gram-negative bacteria compared to earlier generations. They have a broader spectrum of activity and increased potency against some resistant strains.
Fourth-generation fluoroquinolones: The fourth-generation fluoroquinolones represent the most recent development in this class of antibiotics. Examples include gemifloxacin and trovafloxacin (withdrawn from the market due to safety concerns). Fourth-generation fluoroquinolones offer expanded coverage against Gram-positive bacteria, including strains resistant to earlier generations.
STRUCTURE, ACTIVITY AND RELATIONSHIP OF FLUOROQUINOLONE [28-30]
The structure-activity relationship (SAR) of fluoroquinolones refers to the relationship between the chemical structure of these antibiotics and their pharmacological activity, including potency, spectrum of activity, and pharmacokinetic properties. Small modifications to the structure of fluoroquinolones can significantly influence their antimicrobial activity and other properties (Figure 1).
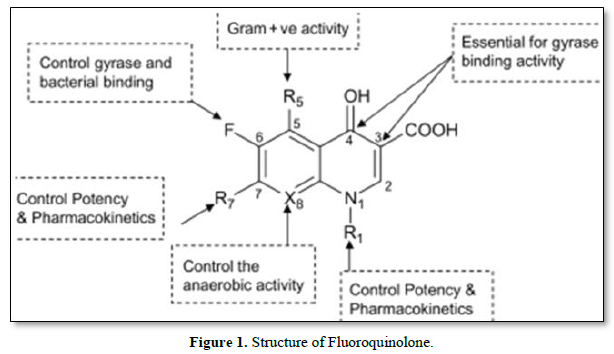
Here are some key structural features and their impact on the activity of fluoroquinolones:
Fluorine Atom: The presence of a fluorine atom at the C-6 position is a defining characteristic of fluoroquinolones. This fluorine atom enhances the potency and broad-spectrum activity of the drug by increasing its affinity for the target enzymes, DNA gyrase, and topoisomerase IV.
Substituents at the C-7 position: Various substituents at the C-7 position of the fluoroquinolone molecule can influence its potency and spectrum of activity. For example, the introduction of a piperazinyl group (as in ciprofloxacin) enhances the activity against Gram-negative bacteria. On the other hand, the presence of bulky or hydrophobic groups may lead to increased activity against Gram-positive bacteria.
Substituents at the C-1 position: Modifications at the C-1 position can affect both antimicrobial activity and pharmacokinetics. The presence of a cyclopropyl or cyclopropyl methyl group at C-1 (as in ciprofloxacin and levofloxacin) improves the activity against Gram-positive bacteria and enhances oral bioavailability.
Substituents at the N-1 position: Substituents at the N-1 position, such as an alkyl group or a cyclopropyl methyl group (as in levofloxacin), can influence the activity against Gram-positive bacteria and increase the drug's half-life.
Quinolone Core Structure: The quinolone core structure consists of a bicyclic ring system, and variations in this core structure can affect the potency and other properties of fluoroquinolones. Modifications to the core structure can lead to changes in the binding affinity to the target enzymes and alter the drug's pharmacokinetic profile.
BIOLOGICAL SIGNIFICANCE OF FLUOROQUINOLONE [31,32]
Fluoroquinolones have demonstrated a high potential in various medical applications due to their unique properties and broad-spectrum antimicrobial activity. Here are some potential applications of fluoroquinolones:
Treatment of bacterial infections: Fluoroquinolones are widely used in the treatment of bacterial infections, including respiratory tract infections, urinary tract infections, skin and soft tissue infections, bone and joint infections, and gastrointestinal infections. Their broad-spectrum activity and ability to penetrate various tissues make them effective against a wide range of pathogens.
Management of drug-resistant infections: Fluoroquinolones have been crucial in managing drug-resistant bacterial infections, including those caused by multidrug-resistant Gram-negative bacteria, such as Pseudomonas aeruginosa and Acinetobacter baumannii. They have shown effectiveness against methicillin-resistant Staphylococcus aureus (MRSA) and other resistant strains, providing treatment options when other antibiotics fail.
Tuberculosis treatment: Fluoroquinolones, such as levofloxacin and moxifloxacin, have been used in the treatment of drug-resistant tuberculosis (TB). They are part of the second-line drugs used in multidrug-resistant TB (MDR-TB) and extensively drug-resistant TB (XDR-TB) regimens. Fluoroquinolones have shown good activity against Mycobacterium tuberculosis, helping to improve treatment outcomes for resistant strains.
Traveler's diarrhea prevention and treatment: Fluoroquinolones, particularly ciprofloxacin, have been used as prophylaxis and treatment for traveler's diarrhea, a common condition experienced by travelers visiting regions with a higher risk of bacterial gastrointestinal infections. Fluoroquinolones have shown efficacy in reducing the duration and severity of symptoms.
Anthrax treatment: Fluoroquinolones, specifically ciprofloxacin and levofloxacin, have been recommended for the treatment and post-exposure prophylaxis of anthrax, a severe bacterial infection caused by Bacillus anthracis. Fluoroquinolones are effective against anthrax and are considered essential drugs in the management of this potentially life-threatening condition.
Prophylaxis in high-risk situations: Fluoroquinolones are sometimes used as prophylactic antibiotics in certain high-risk situations, such as surgical procedures, to prevent postoperative infections. Their broad-spectrum activity and good tissue penetration make them valuable in reducing the risk of infection in vulnerable individuals.
MECHANISM OF ACTION OF FLUOROQUINOLONE
Fluoroquinolones are a class of antibiotics that are effective against a wide range of bacterial infections, including tuberculosis. Their mechanism of action involves targeting enzymes involved in DNA replication and synthesis, thereby interfering with the bacterial DNA replication process. This disruption ultimately leads to the inhibition of bacterial growth and the death of the bacteria.
General overview of the mechanism of action of fluoroquinolones [33-35]:
DNA Gyrase Inhibition: Fluoroquinolones primarily target two key bacterial enzymes called DNA gyrase (topoisomerase II) and topoisomerase IV. These enzymes are involved in the replication, transcription, and repair of DNA. Fluoroquinolones bind to the DNA gyrase subunits and inhibit their activity, preventing them from properly managing DNA supercoiling. This leads to the accumulation of DNA breaks and prevents the proper separation of DNA strands during replication and transcription.
DNA Replication Interference: By inhibiting DNA gyrase, fluoroquinolones interfere with the unwinding and separation of DNA strands, which is necessary for DNA replication. This disruption hinders the synthesis of new DNA strands and prevents the bacteria from reproducing.
DNA Repair Impairment: Fluoroquinolones also interfere with the repair of DNA damage within the bacteria. DNA gyrase and topoisomerase IV play crucial roles in repairing DNA breaks, and their inhibition by fluoroquinolones prevents efficient DNA repair processes. This leads to the accumulation of DNA damage, further hindering bacterial survival.
CONCLUSION
Tuberculosis remains a major global health concern, particularly in resource-limited settings. Continued research, improved diagnostics, and access to effective treatment are essential for reducing the burden of tuberculosis and achieving global tuberculosis elimination goals. Tuberculosis treatment relies on a combination of multiple antitubercular drugs to achieve bactericidal activity, prevent the development of drug resistance, and reduce treatment duration. The standard regimen for drug-sensitive tuberculosis consists of an initial phase of four drugs (isoniazid, rifampin, pyrazinamide, and ethambutol) followed by a continuation phase of isoniazid and rifampin. Directly Observed Treatment, Short-Course (DOTS) is the recommended strategy to ensure treatment adherence. The emergence of drug-resistant tuberculosis, particularly multidrug-resistant tuberculosis (MDR-TB) and extensively drug-resistant tuberculosis (XDR-TB), poses a significant global health challenge. Diagnosis of drug resistance is crucial for appropriate treatment selection, and newer drugs and regimens, such as bedaquiline and delamanid, have been introduced to tackle drug-resistant tuberculosis. Fluoroquinolones are a class of antibiotics with a broad spectrum of activity against various bacterial infections. Additionally, the quinolone core structure plays a vital role in determining the binding affinity to the target enzymes.
- Sandhu G (2011) Tuberculosis: Current situation, challenges and overview of its control programs in India. J Global Infect Dis 3(2): 143.
- World Health Organization (2022) Global Tuberculosis Report 2022.
- World Health Organization (2021) Global Tuberculosis Report 2021.
- World Health Organization (2020) Global Tuberculosis Report 2020.
- Natarajan A, Beena PM, Devnikar A, Mali S (2020) A systemic review on tuberculosis. Ind J Tuberc 67(3): 295-311.
- Sharma SK, Mohan A (2013) Tuberculosis: From an incurable scourge to a curable disease. Journey over a Millennium. Ind J Med Res 137(3): 455-493.
- Boom WH, Schaible UE, Achkar JM (2021) The knowns and unknowns of latent Mycobacterium tuberculosis J Clin Invest 131(3): e136222.
- Chakaya J, Khan M, Ntoumi F, Aklillu E, Fatima R, et al. (2021) Global Tuberculosis Report 2020 - Reflections on the Global TB burden, treatment and prevention efforts. Int J Infect Dis 113(Suppl 1): S7-S12.
- Sotgiu G, Centis R, D’ambrosio L, Migliori GB (2015) Tuberculosis Treatment and Drug Regimens. Cold Spring Harbor Perspectives in Medicine 5(5):
- Mase SR, Chorba T (2019) Treatment of Drug-Resistant Tuberculosis. Clin Chest Med 40(4): 775-795.
- Pham TDM, Ziora Z, Blaskovich M (2019) Quinolone Antibiotics. Med Chem Comm 10: 1719-1739.
- Emami S, Shafiee A, Foroumadi A (2005) Quinolones: Recent structural and clinical Developments. Iran J Pharm Res 3: 123-125.
- Emami S, Shafiee A, Foroumadi A (2005) Quinolones: Recent structural and clinical developments. Iran J Pharm Res 3: 123-136.
- Rabahi MF, Silva Júnior JLR da, Ferreira ACG, Tannus-Silva DGS, Conde MB (2017) Tuberculosis treatment. Jornal Brasileiro de Pneumologia 43(6): 472-486.
- Méchaï F, Cordel H, Guglielmetti L, Aubry A, Jankovic M, et al. (2020) Management of Tuberculosis: Are the Practices Homogeneous in High-Income Countries? Front Public Health 8: 443.
- Migliori GB, Sotgiu G, Rosales-Klintz S, Centis R, D’Ambrosio L, et al. (2018) ERS/ECDC Statement: European Union standards for tuberculosis care, 2017 update. Eur Respir J 51(5):
- Bendre AD, Peters PJ, Kumar J (2021) Tuberculosis: Past, present and future of the treatment and drug discovery research. Curr Res Pharmacol Drug Disc 2:
- Zhanel GG, Walkty A, Vercaigne L, Karlowsky JA, Embil J, et al. (1999) The New Fluoroquinolones: A Critical Review. Can J Infect Dis 10(3): 207-238.
- Jain SD, Gupta AK (2021) Chemistry of Fluoroquinones in the Management of Tuberculosis (TB): An Overview. Asian J Pharm Res 11(1): 55-59.
- Mogle BT, Steele JM, Thomas SJ, Bohan KH, Kufel WD (2018) Clinical review of delafloxacin: A novel anionic fluoroquinolone. J Antimicrob Chemother 73(6): 1439-1451.
- Naeem A, Badshah SL, Muska M, Ahmad N, Khan K (2016) The Current Case of Quinolones: Synthetic Approaches and Antibacterial Activity. Molecules (Basel, Switzerland) 21(4): 268.
- Shandil RK, Jayaram R, Kaur P, Gaonkar S, Suresh BL, et al. (2007) Moxifloxacin, ofloxacin, sparfloxacin, and ciprofloxacin against Mycobacterium tuberculosis: Evaluation of in vitro and pharmacodynamic indices that best predict in vivo Antimicrob Agents Chemother 51(2): 576-582.
- Velyati AA, Masjedi MR, Farnia P, Tabarsi P, Ghanavi J, et al. (2009) Emergence of new forms of totally drug-resistant tuberculosis bacilli: Super extensively drug-resistant tuberculosis or totally drug-resistant strains in Iran. Chest 136 (2): 420-425.
- Brar RK, Jyoti U, Patil RK, Patil HC (2020) Fluoroquinolone antibiotics: An overview. Adesh Uni J Med Sci Res 2(1): 26-30.
- Daniel DB, Ramachandran G, Swaminathan S (2017) The challenges of pharmacokinetic variability of first-line anti-TB drugs. Expert Rev Clin Pharmacol 10(1): 47-58.
- Asif M, Siddiqui AA, Husain A (2013) Quinolone derivatives as antitubercular drugs. Med Chem Res 22(3): 1029-1042.
- Conde MB, Efron A, Loredo C, De Souza GR, Graca NP, et al. (2009) Moxifloxacin versus ethambutol in the initial treatment of tuberculosis: a double blind, randomized, controlled phase II trial. Lancet 373: 1183-1189.
- Blondeau JM (2004) Fluoroquinolones: Mechanism of action, classification, and development of resistance. Surv Ophthalmol 49(2): S73-S78.
- Pranger AD, van der Werf TS, Kosterink JGW, Alffenaar JWC (2019) The Role of Fluoroquinolones in the Treatment of Tuberculosis in 2019. Drugs 79(2): 161-171.
- Naber KG, Adam D (1998) Classification of fluoroquinolones. Int J Antimicrob Agents 10(4): 255-257.
- Aldred KJ, Kerns RJ, Osheroff N (2014) Mechanism of Quinolone Action and Resistance. Biochemistry 53(10): 1565-1574.
- Bush NG, Diez-Santos I, Abbott LR, Maxwell A (2020) Quinolones: Mechanism, Lethality and Their Contributions to Antibiotic Resistance. Molecules 25(23): 5662.
- Hooper DC, Jacoby GA (2016) Topoisomerase Inhibitors: Fluoroquinolone Mechanisms of Action and Resistance. Cold Spring Harb Perspect Med 6(9):
- Zhao X, Xu C, Domagala J, Drlica K (1997) DNA topoisomerase targets of the fluoroquinolones: A strategy for avoiding bacterial resistance. Proc Natl Acad Sci 94(25): 13991-13996.
- Mustaev A, Malik M, Zhao X, Kurepina N, Luan G, et al. (2014) Fluoroquinolone-Gyrase- DNA Complexes. J Biol Chem 289(18): 12300-12312.